Diabetes mellitus is a chronic metabolic disorder that has emerged as a major public health concern worldwide, affecting millions and placing a significant burden on healthcare systems. Despite advancements in care, the growing prevalence of both type 1 and type 2 diabetes underscores the need for more effective diagnostic and therapeutic strategies. Type 1 diabetes, an autoimmune condition characterized by the destruction of insulin-producing beta cells, continues to rise globally especially among children and young adults. On the other hand, type 2 diabetes often linked to lifestyle and metabolic factors, frequently remains undiagnosed until complications develop.
While maintaining near-normal blood glucose levels such as fasting plasma glucose between 90–130 mg/dL and HbA1c below 7% remains a cornerstone of diabetes management, a singular focus on glycemic control is no longer sufficient. Comprehensive care must also address coexisting conditions such as hypertension and dyslipidemia to reduce the risk of long-term complications including cardiovascular disease, kidney failure, neuropathy and retinopathy.
Early and accurate diagnosis plays a pivotal role in managing diabetes effectively. Although screening has not consistently shown short-term mortality benefits, long-term studies suggest that early identification paired with lifestyle and pharmacologic interventions may delay disease progression and prevent complications. Additionally, recent technological advances such as continuous glucose monitors and portable diagnostic devices are transforming how diabetes is detected and monitored, offering patients more control and clinicians better data for decision-making.
This article explores the evolving landscape of diabetes diagnosis and treatment, encompassing both pharmacological and non-pharmacological approaches, with an emphasis on personalized care and sustainable lifestyle strategies.
Diagnosis
Diagnosing diabetes mellitus involves identifying persistent hyperglycemia and accurately classifying the type of diabetes to guide appropriate treatment. Current diagnostic protocols, as endorsed by leading guidelines such as those from the American Diabetes Association (ADA), rely on standardized glucose and glycated hemoglobin (A1C) testing. Diagnosis may be established using any of the following criteria:
1. Fasting Plasma Glucose (FPG): ≥ 126 mg/dL (7.0 mmol/L) after no caloric intake for at least 8 hours.
2. 2-Hour Plasma Glucose (2-h PG): ≥ 200 mg/dL (11.1 mmol/L) following a 75-gram oral glucose tolerance test (OGTT).
3. Random Plasma Glucose (RPG): ≥ 200 mg/dL (11.1 mmol/L) in individuals with symptoms of hyperglycemia.
4. Hemoglobin A1C (HbA1c): ≥ 6.5% (48 mmol/mol), measured using NGSP-certified and DCCT-standardized methods.
In the absence of a hyperglycemic crisis or classic symptoms (such as polyuria, polydipsia or unexplained weight loss), a second abnormal result either from the same or a different test is required for confirmation.
Once hyperglycemia is confirmed, determining the type of diabetes is essential. Type 1 diabetes (T1DM) often presents in childhood or adolescence with abrupt onset, low body weightand may include ketosis or diabetic ketoacidosis. Laboratory markers such as the presence of pancreatic autoantibodies (e.g., GAD, IA-2) and low C-peptide levels support a diagnosis of T1DM.
In contrast, Type 2 diabetes (T2DM) generally develops later in life, particularly after puberty and is commonly associated with overweight or obesity, signs of insulin resistance (such as acanthosis nigricans) and a strong familial tendency. C-peptide levels in T2DM are usually normal or elevated and autoantibodies are typically absent.
Additionally, rare forms like monogenic diabetes (e.g., MODY) should be considered in younger individuals with non-insulin-dependent diabetes, preserved beta-cell function, absence of autoimmunity and a clear autosomal dominant inheritance pattern. Genetic testing is required to confirm such diagnoses.
Accurate diagnosis and classification are foundational for initiating effective treatment plans, tailoring monitoring strategies and preventing long-term complications associated with diabetes.1-4
Management of Diabetes
Modern diabetes management embraces a multi-pronged, individualized strategy emphasizing early diagnosis, patient-centered care and therapeutic diversity. Optimal outcomes are achieved by combining lifestyle modification, medical nutrition therapy and evidence-based pharmacological interventions, supported by emerging tools like nanotechnology and gene therapy.4-7
1. Non-Pharmacological Treatment
a. Lifestyle Modification:
Lifestyle intervention is foundational to both prevention and management of Type 2 Diabetes Mellitus (T2DM). Key strategies include:
● Physical Activity: Minimum 150 minutes/week of moderate aerobic activity.
● Dietary Changes: Low glycemic index, high-fiber diet rich in vegetables, fruits, whole grains and lean proteins.
● Weight Reduction: Aiming for 5–10% reduction in overweight individuals.
● Smoking Cessation
● Moderation of alcohol intake
● Internet-Based Interventions: Digital tools and telehealth platforms support:
○ Real-time coaching and reminders
○ Diet and glucose tracking
○ Peer support forums
Studies show these tools improve adherence and glycemic control, especially in younger and tech-literate populations.
b. Medical Nutrition Therapy (MNT):
MNT is a personalized nutritional care delivered by registered dietitian nutritionists. Its goals include:
● Optimizing glycemic control
● Managing comorbidities (e.g., dyslipidemia, hypertension)
● Preventing complications
c. Gestational Diabetes Considerations:
● Carbohydrate intake ≥175 g/day
● Avoid ketogenesis
● Balanced energy intake to support fetal growth
d. Nanotechnology in Diabetes Care:
Nanomedicine improves diagnosis, monitoring and drug delivery:
● Glucose Monitoring: Nano-biosensors are tiny devices constructed using advanced nanomaterials such as carbon nanotubes or gold nanoparticles. These sensors are designed to detect glucose levels in real-time from body fluids like sweat, saliva or tears offering a non-invasive alternative to traditional methods. The major advantage of these nano-biosensors is the elimination of frequent finger pricks, providing patients with more comfort and the ability to continuously monitor their glucose levels.
● Insulin Delivery: Nanomedicine enables innovative insulin delivery methods using nanoparticle formulations that can encapsulate insulin for oral or inhaled administration. These formulations help insulin survive harsh conditions in the digestive system or lungs. Additionally, closed-loop nano-pump systems are being developed to mimic the function of a healthy pancreas. These smart devices can automatically sense blood glucose levels and release insulin accordingly. The goal is to achieve precise glucose control, reduce the frequency of injections and enhance overall blood sugar management.
● Early Diagnosis: Magnetic nanoparticles (MNPs) are being utilized in MRI imaging to visualize pancreatic β-cells, the insulin-producing cells of the pancreas. This technology allows for the early detection of β-cell loss or dysfunction even before the onset of overt diabetes. Early diagnosis using nanomedicine could enable timely interventions and potentially prevent disease progression.
● Gene & Cell Therapy Carriers: Nanoparticles also serve as effective carriers for delivering genes or therapeutic cells directly to targeted tissues. This targeted approach enhances the precision of treatment, minimizes side effects and paves the way for advanced disease-modifying therapies. These may include gene editing technologies or methods to regenerate β-cells, offering promising new avenues in the long-term management and potential cure of diabetes.
e. Gene Therapy:
Gene therapy targets underlying genetic dysfunction in both T1DM and T2DM.
● T1DM: In T1DM, the immune system mistakenly attacks and destroys pancreatic β-cells, which produce insulin. Gene therapy in T1DM focuses on two main strategies:
○ Enhancing β-cell regeneration: Introducing or activating genes that can stimulate the regrowth or regeneration of insulin-producing cells.
○ Modulating autoimmune responses: Delivering genes that suppress or regulate the immune system to prevent further destruction of β-cells.
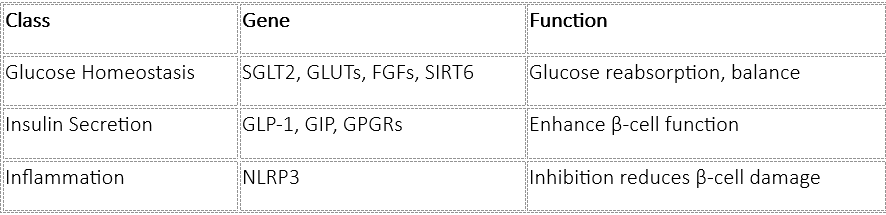
● T2DM: T2DM is driven by insulin resistance and progressive β-cell dysfunction. Gene therapy here targets pathways involved in insulin sensitivity, secretion, and inflammation. Several specific genes have been identified as promising targets:
2. Pharmacological Treatment
Pharmacotherapy is essential when lifestyle modifications are insufficient. Drug selection is based on:
● Patient age, weight, comorbidities
● Risk of hypoglycemia
● Cost and patient preferences
2. First-Line Agent: Metformin
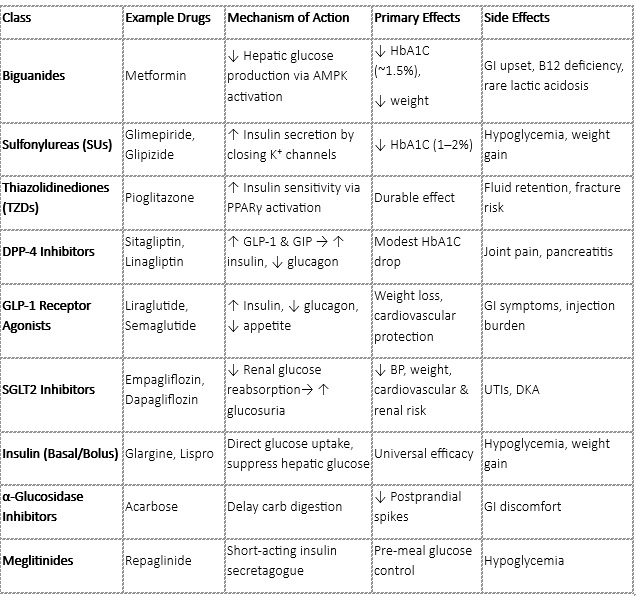
Metformin is the first-line pharmacologic agent for type 2 diabetes mellitus (T2DM) and exerts its primary effect by suppressing hepatic gluconeogenesis, thereby reducing the liver’s production of glucose. Additionally, it increases insulin sensitivity in peripheral tissues, enhancing glucose uptake. This dual action helps to lower blood glucose levels effectively.
The benefits of metformin are well established. It offers cardiovascular protection, as demonstrated in long-term studies like the UKPDS (United Kingdom Prospective Diabetes Study).
Unlike many other antidiabetic agents, metformin is weight neutral or may even lead to modest weight loss, which is especially beneficial for overweight or obese individuals with T2DM. It also carries a low risk of causing hypoglycemia, making it a safe option when used alone.
However, there are some limitations to its use. Metformin should be avoided in patients with severely impaired kidney function, particularly when the estimated glomerular filtration rate (eGFR) is below 30 mL/min/1.73 m², due to the risk of lactic acidosis. Gastrointestinal side effects such as nausea, diarrhea and abdominal discomfort are also relatively common but often transient.
In terms of glycemic control, metformin typically reduces HbA1c levels by approximately 1% to 1.5%, making it a potent and cost-effective foundation for diabetes management.
3. Combination and Advanced Therapy:
When monotherapy fails (HbA1c remains above target), combination therapy is recommended:
● Dual Therapy: Metformin + SGLT2i/GLP-1 RA preferred in cardiovascular or renal disease.
● Triple Therapy: Add DPP-4inhibitors, Sulfonylureas or basal insulin
● Insulin Therapy:
○ Start with basal insulin (e.g., glargine)
○ Add prandial insulin if needed
4. Future Therapeutics and Trends:
a. Oral GLP-1 Agonists: A major advancement in diabetes treatment is the development of oral GLP-1 receptor agonists such as oral semaglutide (Rybelsus®). Traditionally administered via injection, GLP-1 agonists improve glycemic control and promote weight loss. The oral formulation enhances patient adherence by offering a more convenient, non-invasive option without compromising efficacy.
b. Dual Agonists: Tirzepatide, a novel dual agonist targeting both GLP-1 and GIP receptors has shown remarkable results in clinical trials. It provides superior glucose-lowering and weight-reduction effects compared to single agonists. By acting on two incretin pathways, it enhances insulin secretion, reduces appetite and delays gastric emptying, offering a multifaceted approach to T2DM management.
c. Smart Insulins: These are glucose-responsive insulin formulations that become active only when blood glucose levels rise. This “on-demand” insulin delivery mimics the physiological function of pancreatic β-cells, potentially minimizing the risk of hypoglycemia while offering more precise glycemic control.
d. Beta-cell Regenerative Therapies: Researchers are developing small molecules that can stimulate the regeneration or proliferation of insulin-producing β-cells. These therapies aim to restore endogenous insulin production, particularly beneficial in early T1DM or advanced T2DM with declining β-cell function.
e. CRISPR/Cas9 Gene Editing: Gene editing technologies like CRISPR/Cas9 are being explored to modify or correct genes associated with diabetes susceptibility. This innovative approach could potentially reverse insulin resistance or β-cell dysfunction at the genetic level, offering the possibility of a long-term cure or disease modification in diabetes.
Conclusion:
Diabetes mellitus remains a complex and multifaceted global health challenge, demanding a comprehensive and personalized approach to diagnosis, management and prevention. Advances in diagnostic technologies combined with evolving pharmacological and non-pharmacological therapies have significantly improved patient outcomes. However, the rising prevalence of both type 1 and type 2 diabetes calls for continued innovation and vigilance.
Modern management strategies emphasize early detection, lifestyle modification and individualized treatment plans integrating cutting-edge tools such as nanotechnology, gene therapy and novel pharmacotherapies. These innovations hold promise not only for improved glycemic control but also for addressing the underlying pathophysiology and preventing long-term complications.
As research progresses, emerging therapies like beta-cell regeneration, smart insulins and gene editing technologies such as CRISPR/Cas9 could revolutionize diabetes care by potentially altering disease progression or offering curative options. Ultimately, a multidisciplinary, patient-centered model combined with ongoing scientific advancements will be key to combating this silent epidemic and improving quality of life for millions affected worldwide.
References
- American Diabetes Association Professional Practice Committee; 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care1 January 2024; 47 (Supplement_1): S20–S42. https://diabetesjournals.org/care/article/47/Supplement_1/S20/153954/2-Diagnosis-and-Classification-of-Diabetes
- American Diabetes Association Professional Practice Committee; 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care1 January 2025; 48 (Supplement_1): S27–S49. https://diabetesjournals.org/care/article/48/Supplement_1/S27/157566/2-Diagnosis-and-Classification-of-Diabetes
- World Health Organization 2020. Diagnosis and Management of Type 2 Diabetes. Accessed on May 27, 2025. https://iris.who.int/bitstream/handle/10665/331710/WHO-UCN-NCD-20.1-eng.pdf?sequence=1
- Serbis A, Giapros V, Kotanidou EP, Galli-Tsinopoulou A, Siomou E. Diagnosis, treatment and prevention of type 2 diabetes mellitus in children and adolescents. World J Diabetes2021; 12(4): 344-365. https://www.wjgnet.com/1948-9358/full/v12/i4/344.htm
- Aloke C, Egwu CO, Aja PM, Obasi NA, Chukwu J, Akumadu BO, Ogbu PN, Achilonu I. Current Advances in the Management of Diabetes Mellitus. Biomedicines. 2022 Sep 29;10(10):2436. https://pmc.ncbi.nlm.nih.gov/articles/PMC9599361/#sec4-biomedicines-10-02436
- Thrasher, James. Pharmacologic Management of Type 2 Diabetes Mellitus: Available Therapies. The American Journal of Medicine. 2017;130(6), S4 – S17. https://www.amjmed.com/article/s0002-9343(17)30457-6/fulltext
- American Diabetes Association Professional Practice Committee; Summary of Revisions: Standards of Care in Diabetes—2025. Diabetes Care1 January 2025; 48 (Supplement_1): S6–S13. https://diabetesjournals.org/care/article/48/Supplement_1/S6/157564/Summary-of-Revisions-Standards-of-Care-in-Diabetes