According to the World Health Organization (WHO), diabetes has witnessed a dramatic surge in global prevalence, rising from approximately 200 million cases in 1990 to an estimated 830 million by 2022. This upward trend has been especially steep in low- and middle-income countries, where access to timely diagnosis and consistent treatment remains critically inadequate. Alarmingly, more than half of the individuals living with diabetes were not receiving any medication for the condition as of 2022 reflecting serious global disparities in healthcare coverage.
Diabetes is far more than a chronic blood sugar disorder; it is a leading cause of numerous serious health complications. WHO reports that it contributes to blindness, kidney failure, heart attacks, strokes and lower limb amputations. Moreover, it is a major contributor to mortality worldwide. Given the growing impact of this disease on public health and individual well-being, it is essential to understand diabetes in greater depth. This article delves into the causes, symptoms, pathophysiology, risk factors and complications of diabetes, providing a deeper understanding of the condition.
What is Diabetes Mellitus?
Diabetes Mellitus (DM) is a chronic, metabolic disorder characterized by persistent hyperglycemia (elevated blood glucose levels) resulting from impaired insulin secretion, defective insulin action or a combination of both. Insulin, an essential anabolic hormone produced by the pancreas, plays a key role in regulating the metabolism of carbohydrates, fats and proteins. When the body cannot produce sufficient insulin or fails to use it effectively, glucose accumulates in the bloodstream leading to various acute and long-term health complications.
Over time, chronic hyperglycemia can damage multiple organ systems, particularly the eyes (retinopathy), kidneys (nephropathy), nerves (neuropathy) and blood vessels, significantly increasing the risk of cardiovascular diseases. Diabetes is broadly classified into several types with Type 1, Type 2 and gestational diabetes being the most common.1-5
What Is Prediabetes?
Prediabetes also known as borderline diabetes, is a reversible medical condition in which blood glucose levels are higher than normal but not yet high enough to meet the diagnostic criteria for type 2 diabetes. It is also referred to as impaired fasting glucose, impaired glucose tolerance, impaired glucose regulation and non-diabetic hyperglycaemia. It represents a critical early warning stage in the development of diabetes and signals impaired glucose regulation in the body.
Prediabetes is often silent, with no noticeable symptoms. Many individuals may only discover they have it through routine blood tests. However, if symptoms such as frequent urination, excessive thirst, fatigue or unexplained weight loss begin to appear, type 2 diabetes may have already developed.
There are three key indicators used to identify prediabetes:
1. Impaired Fasting Glucose (IFG): This refers to elevated blood glucose levels after an overnight fast, typically in the range of 100–125 mg/dL (5.6–6.9 mmol/L). It suggests the body is beginning to lose its ability to regulate glucose properly during periods of fasting.
2. Impaired Glucose Tolerance (IGT): This is detected using an Oral Glucose Tolerance Test (OGTT), where blood glucose levels measured 2 hours after consuming a glucose-rich drink fall between 140–199 mg/dL (7.8–11.0 mmol/L). This reflects how the body handles glucose after eating.
3. Elevated Glycated Hemoglobin (HbA1c): HbA1c reflects average blood glucose over the past 2–3 months. A level between 6.0% and 6.4% indicates prediabetes. It’s a convenient and reliable screening tool widely used in clinical practice.
Prediabetes is a high-risk state for developing type 2 diabetes, cardiovascular disease and other metabolic disorders. If left unaddressed, many individuals with prediabetes will progress to full-blown diabetes within 5 to 10 years. However, the good news is that early detection and proactive intervention through lifestyle changes such as improved diet, regular physical activity and weight management can prevent or delay the onset of diabetes.6,7,8
Classification of Diabetes Mellitus
Diabetes mellitus classified into several types:2,9
1.Type 1 Diabetes (Insulin Dependent Diabetes Mellitus (IDDM))
Type 1 diabetes (T1D) can start developing even before any clear signs of abnormal insulin levels appear. The ability of the pancreas’s beta cells to produce insulin begins to decline gradually, often starting at least two years before diagnosis. Early on, the first quick release of insulin after meals gets weaker, while the later insulin release might increase for a while to make up for it. After diagnosis, insulin production keeps dropping, usually faster in the first year than in the second. Over time, the pancreas stops making insulin almost completely. Even if blood sugar seems normal, changes like unstable sugar levels and signs of dysglycemia may point to early T1D. Monitoring blood sugar and C-peptide levels in people at risk can help detect the disease early.
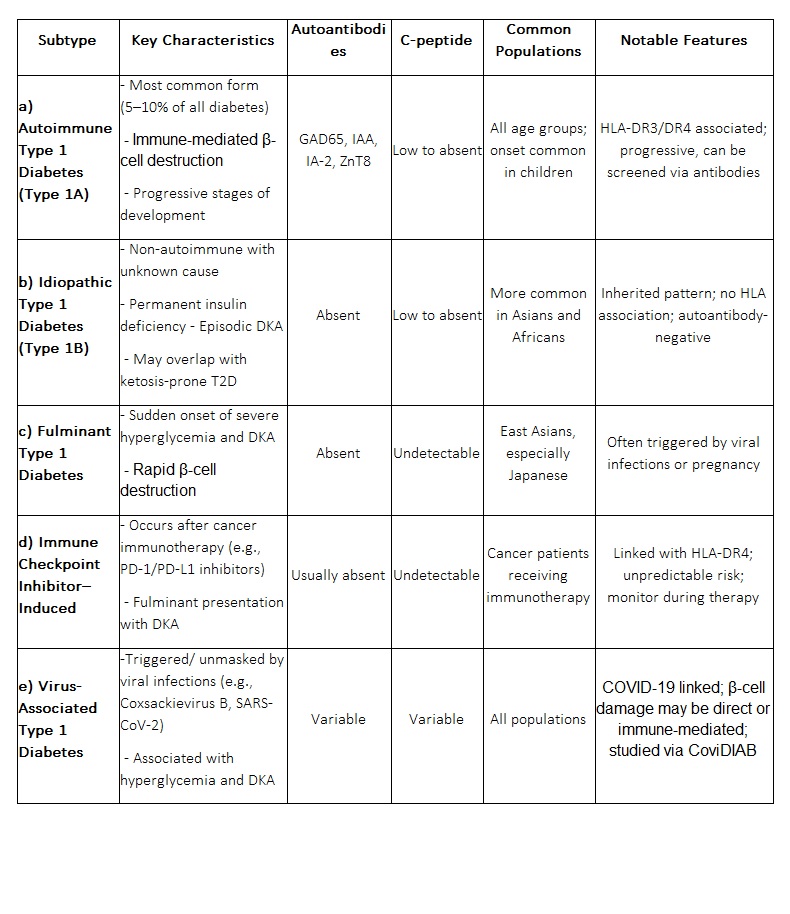
a) Autoimmune Type 1 Diabetes (Type 1A):
This is the most common form of T1D accounting for about 5–10% of all diabetes cases. It is caused by immune-mediated destruction of β-cells and is characterized by the presence of autoantibodies against pancreatic islet cells, such as:
● Glutamic acid decarboxylase (GAD65)
● Insulin autoantibodies (IAA)
● Islet antigen 2 (IA-2) and IA-2β
● Zinc transporter 8 (ZnT8)
The disease has strong associations with certain HLA genes (especially DR3-DQ2 and DR4-DQ8) and genetic screening is often used in research to identify individuals at high risk.
The progression of autoimmune T1D occurs in stages:
● Stage 1: Presence of two or more autoantibodies with normal blood glucose levels.
● Stage 2: Autoantibodies with dysglycemia (abnormalities in blood glucose levels).
● Stage 3: Symptomatic diabetes with hyperglycemia or diabetic ketoacidosis (DKA).
The rate of β-cell destruction varies, being rapid in children and slower in adults. Although this form often appears in childhood, it can develop at any age.
b) Idiopathic Type 1 Diabetes (Type 1B):
Idiopathic T1D is a non-autoimmune form with unknown etiology. These patients lack the typical autoantibodies seen in autoimmune T1D but still have permanent insulin deficiency and are prone to episodic diabetic ketoacidosis. It is more commonly observed in individuals of Asian or African descent and is often inherited. Some cases may overlap with ketosis-prone type 2 diabetes especially in those without HLA associations.
c) Fulminant Type 1 Diabetes: This is a rare and rapidly progressing variant characterized by sudden onset of hyperglycemia and diabetic ketoacidosis. Key features include:
● Undetectable C-peptide levels (indicating absent insulin secretion)
● Very high blood glucose levels (>288 mg/dL)
● Absence of autoantibodies
Fulminant T1D is most commonly reported in East Asian populations particularly in Japan and may be triggered by viral infections or immune responses. It has also been reported during pregnancy.
d) Immune Checkpoint Inhibitor–Induced Diabetes:
A novel form of T1D has emerged as an immune-related adverse event in patients receiving checkpoint inhibitors (e.g., anti-PD-1/PD-L1) for cancer therapy. It presents as fulminant diabetes with diabetic ketoacidosis and undetectable C-peptide but often lacks autoantibodies. High-risk HLA-DR4 is present in most affected individuals. Risk is unpredictable by family history or antibody status, making awareness and monitoring essential for patients on these therapies.
e) Virus-Associated Type 1 Diabetes:
Viral infections such as Coxsackievirus B and SARS-CoV-2 (COVID-19) have been implicated in triggering or unmasking T1D. COVID-19, in particular, has been linked to increased cases of hyperglycemia, diabetic ketoacidosis and new-onset diabetes. Possible mechanisms include:
● Direct β-cell damage by the virus
● Immune-mediated destruction
● Inflammatory cytokine storm
A global registry named CoviDIAB has been created to investigate these associations further.
2. Type 2 Diabetes (Non-Insulin Dependent Diabetes Mellitus (NIDDM))
In type 2 diabetes (T2D), one of the core issues is impaired insulin secretion by the pancreatic β-cells. Under normal conditions, insulin secretion increases as insulin sensitivity decreases, maintaining normal blood glucose levels. This balance between insulin secretion and sensitivity is quantified by the “disposition index.” In individuals with T2D, the disposition index is reduced, meaning the pancreas cannot adequately compensate for insulin resistance. Although some insulin-resistant patients may have high insulin levels, these are still insufficient relative to the degree of resistance they exhibit.
A key early defect in T2D is a reduced or absent early-phase insulin response to glucose which is crucial for controlling post-meal blood sugar spikes. Additionally, individuals with T2D often show elevated proinsulin-to-insulin ratios, indicating dysfunctional insulin processing and β-cell stress. Over time, β-cell function continues to decline leading to progressively worsening hyperglycemia and making the disease increasingly difficult to manage.
3. Gestational Diabetes
Gestational Diabetes Mellitus (GDM) is defined as glucose intolerance first recognized during pregnancy, although it often reflects previously undiagnosed type 2 diabetes due to insufficient routine screening before conception. GDM is associated with β-cell dysfunction and insulin resistance and poses significant short- and long-term risks for both mother and fetus. These include pre-eclampsia, preterm birth, macrosomia (excessive birth weight over 4,000 g causing delivery risk), cesarean delivery and neonatal complications. Offspring of mothers with GDM have an increased risk of developing obesity, glucose intolerance and diabetes later in life.
Major risk factors for GDM include obesity, advanced maternal age, family history of diabetes, polycystic ovary syndrome (PCOS) and sedentary lifestyle. Diagnosis is typically made using fasting plasma glucose (FPG) and a 75-g oral glucose tolerance test (OGTT) between 24–28 weeks of gestation. In high-risk individuals, early screening before 15 weeks is advised, with FPG ≥110 mg/dL or A1C ≥5.9% indicating early glucose dysregulation. Management includes dietary counseling, glucose monitoring and insulin therapy if needed. A1C is convenient but not reliable for GDM screening after 15 weeks due to physiological changes in pregnancy. GDM is a strong predictor of future type 2 diabetes in the mother, necessitating lifelong screening and preventive care.
4. Hybrid Forms of Diabetes
a. Latent Autoimmune Diabetes in Adults (LADA):
LADA is a slow-progressing, autoimmune form of diabetes that initially mimics type 2 diabetes. It is characterized by the presence of pancreatic autoantibodies, particularly glutamic acid decarboxylase (GAD). While initially manageable with oral agents and lifestyle changes, patients often progress more quickly to insulin therapy.
Diagnostic criteria include: age >35 years at onset, positive GAD antibodies and no need for insulin within the first 6–12 months post-diagnosis. A similar variant, latent autoimmune diabetes in youth (LADY) is also recognized.
b. Ketosis-Prone Type 2 Diabetes:
Primarily seen in African American and sub-Saharan African populations, this form presents with acute ketoacidosis and insulin deficiency, resembling T1D. However, patients often recover insulin secretion after glycemic control is restored and may enter remission. Around 90% relapse within 10 years. The cause is not fully understood and neither autoimmune markers nor specific genetic links have been confirmed. Glucose toxicity is thought to cause transient β-cell dysfunction.
5. Other Specific Types of Diabetes
a. Monogenic Diabetes (MODY):
Maturity-Onset Diabetes of the Young (MODY) is a rare inherited form of diabetes resulting from autosomal dominant genetic mutations that impair pancreatic β-cell function. It typically presents before the age of 25, though diagnosis may occur later. MODY is marked by reduced insulin secretion with little or no insulin resistance, especially in non-obese individuals. Common gene mutations include GCK (MODY2), HNF1A (MODY3), and HNF4A (MODY1). Individuals with GCK-MODY usually have mild, stable fasting hyperglycemia and may not require treatment outside of pregnancy. Those with HNF1A or HNF4A mutations often respond well to low-dose sulfonylureas, though insulin may eventually be needed.
Genetic testing is essential for accurate diagnosis and treatment planning. Some MODY forms such as HNF1B mutations are associated with renal cysts and structural abnormalities (e.g., RCAD syndrome).
b. Genetic Defects in Insulin Action:
Genetic mutations in the insulin receptor gene can disrupt insulin signaling, leading to hyperinsulinemia with variable degrees of hyperglycemia. Clinical features may include acanthosis nigricans, virilization and polycystic ovaries. Interestingly, some affected individuals may maintain normal glucose tolerance despite elevated insulin levels.
c. Exocrine Pancreatic Disorders:
Pancreatic Diabetes (Type 3c Diabetes), also known as pancreatogenic diabetes, arises from damage to the pancreas that impairs both endocrine and exocrine functions. It is often misclassified as type 2 diabetes but is distinct in its origin. Causes include pancreatitis (acute or chronic), pancreatic cancer, pancreatic trauma or surgery, cystic fibrosis, hemochromatosis and fibrocalculous pancreatopathy. Postpancreatitis diabetes can occur even after a single episode, with risk increasing in recurrent cases.
A hallmark feature is pancreatic exocrine insufficiency, which can be screened using fecal elastase levels. Unlike type 1 diabetes, autoantibodies are typically absent. Both insulin and glucagon secretion are compromised, often resulting in elevated insulin requirements. Treatment should avoid medications that increase pancreatitis risk such as incretin-based therapies. Insulin therapy is often needed early and in cases of pancreatectomy due to chronic pancreatitis, islet autotransplantation may be considered to preserve some insulin production and reduce insulin dependency.
d. Endocrinopathies: Certain endocrine disorders can lead to insulin resistance and diabetes due to hormone excess. These include acromegaly (growth hormone excess), glucagonoma (glucagon excess), pheochromocytoma (catecholamine excess) and Cushing’s syndrome (cortisol excess). Additionally, hypokalemia from somatostatinomas or aldosteronomas may worsen glucose intolerance.
e. Drug- or Chemical-Induced Diabetes: Some medications (like glucocorticoids and nicotinic acid) and toxins (such as Vacor and pentamidine) may impair insulin action or directly damage pancreatic β-cells, leading to diabetes. Though uncommon, such causes should be considered in vulnerable individuals.
f. Infections: Certain viral infections such as coxsackievirus B, rubella, mumps, adenovirus and cytomegalovirus have been associated with β-cell destruction in the pancreas. In individuals with a genetic predisposition, these viruses can trigger an autoimmune response that mistakenly attacks insulin-producing cells, potentially leading to type 1 diabetes. This mechanism highlights the complex interplay between genetics and environmental triggers in diabetes development.
6. Rare Autoimmune Diabetes Subtypes
a. Stiff-Person Syndrome (SPS):
SPS is a rare autoimmune neurological disorder characterized by progressive muscle stiffness and painful spasms. It is often associated with elevated levels of anti-GAD (glutamic acid decarboxylase) antibodies, which are also found in type 1 diabetes. Because of this shared autoimmunity, individuals with SPS are at an increased risk of developing diabetes, particularly type 1.
b. Anti-Insulin Receptor Antibody Diabetes: In this condition, the body produces autoantibodies against insulin receptors which can block or sometimes mimic insulin action. This unusual mechanism may cause either severe insulin resistance leading to diabetes or paradoxically hypoglycemia. It’s more commonly seen in people with other autoimmune diseases, especially systemic lupus erythematosus (SLE). A typical skin sign seen in affected individuals is acanthosis nigricans, indicating underlying insulin resistance.
Common Symptoms of Diabetes
Diabetes often presents with a variety of signs and symptoms that may appear suddenly (especially in type 1) or develop gradually (especially in type 2). Typical symptoms include:1,10,11
● Frequent urination
● Excessive thirst
● Increased hunger even after eating
● Unexplained weight loss (especially in type 1 diabetes)
● Persistent fatigue
● Blurred vision
● Tingling, pain, or numbness in hands or feet (common in type 2 diabetes)
● Slow-healing cuts or sores
● Frequent infections (genital, urinary tract, skin, oral cavity)
● Dry mouth and burning sensations
● Itching and reactive hypoglycemia
● Acanthosis nigricans (darkened patches of skin)
● Decreased vision
● Sexual dysfunction (e.g., impotence or erectile dysfunction)
Early recognition of these symptoms is crucial for timely diagnosis and management to prevent complications.
Pathophysiology of Type 1 Diabetes Mellitus (T1D)
Type 1 Diabetes Mellitus (T1D) is a chronic autoimmune disorder marked by the progressive destruction of pancreatic β-cells, which are responsible for producing insulin. The pathogenesis is multifactorial, involving a dynamic interplay between genetic susceptibility, environmental triggers, immune dysregulation and cellular stress responses.12,13
1. Initiating Factors: Genetic, Epigenetic and Environmental Interactions Recent research highlights that the development of T1D is not merely the result of autoimmunity but also involves:
● Genetic predisposition (especially certain HLA class II alleles such as HLA-DR3 and DR4),
● Epigenetic modifications,
● Environmental factors (viral infections, dietary components, gut microbiota imbalances).
These components contribute to β-cell immunogenicity and stress, setting the stage for autoimmune destruction.
2. Autoimmune Activation and Immune Cell Infiltration
The disease progresses through a multistep autoimmune process:
● T-cell mediated autoimmunity targets multiple β-cell antigens (e.g., insulin, GAD65, IA-2).
● CD4+ T cells initiate insulitis (inflammatory infiltration of islets), with CD8+ T cells exacerbating β-cell destruction.
● Macrophages and dendritic cells present β-cell antigens, secrete cytokines like IL-1, TNF-α and IFN-γ, further activating cytotoxic T cells.
This immune onslaught leads to the gradual loss of β-cell mass and function.
3. Role of Cytokines and Nitric Oxide in β-cell Damage
Cytokines released by activated immune cells particularly IFN-γ, IL-1 and TNF-α—promote:
● Mitochondrial dysfunction in β-cells,
● Induction of endoplasmic reticulum (ER) stress,
● Nitric oxide (NO) production via inducible NO synthase (iNOS) causing oxidative damage.
This cytokine storm and NO-induced toxicity are central to β-cell apoptosis.
4. Insulitis and β-cell Destruction
Histological studies reveal:
● Islets showing lymphocytic infiltration (insulitis),
● Presence of pseudoatrophic islets (lacking β-cells),
● Residual islets with immune infiltrates coexisting with α, δ and PP cells.
Interestingly, the progression of β-cell destruction is non-linear, often following a relapsing-remitting pattern akin to other autoimmune diseases.
5. Early Phases and Latent Autoimmunity
Autoimmunity can begin years before clinical diagnosis, marked by:
● Emergence of islet autoantibodies (e.g., ICA, GADA, IAA, ZnT8A),
● Limited β-cell damage initially, potentially compensated by regenerative mechanisms or altered antigen expression to evade immunity.
However, as immune regulation fails, β-cell destruction accelerates, leading to overt diabetes.
6. Residual Insulin Production in Long-Standing T1D
Contrary to older beliefs, low but stable levels of insulin production (C-peptide positivity) have been detected in some individuals even years after diagnosis, suggesting:
● Partial β-cell preservation,
● Possible β-cell neogenesis or immune regulation over time.
7. Metabolic Consequences of β-cell Destruction
The loss of insulin leads to severe metabolic disturbances:
a. Glucose Metabolism
● Reduced peripheral glucose uptake (due to lack of GLUT4 activation),
● Increased hepatic glucose output via glycogenolysis and gluconeogenesis,
● Results in Hyperglycemia and glucosuria.
b. Lipid Metabolism
● Uncontrolled lipolysis in adipose tissue,
● Elevated free fatty acids contribute to insulin resistance and serve as substrates for ketogenesis, precipitating diabetic ketoacidosis (DKA).
c. Protein Metabolism
● Muscle proteolysis increases amino acid availability for gluconeogenesis, contributing to weight loss and muscle wasting.
8. α-cell Dysfunction and Glucagon Excess
Despite hyperglycemia, α-cell suppression fails, leading to:
● Inappropriate glucagon secretion,
● Aggravation of hyperglycemia and lipolysis,
● Amplified risk of diabetic ketoacidosis.
Pathogenesis and Pathophysiology of Type 2 Diabetes Mellitus
Type 2 Diabetes Mellitus (T2DM) is a complex, progressive metabolic disorder characterized by insulin resistance and progressive pancreatic β-cell dysfunction, leading to chronic hyperglycemia. The condition develops gradually due to genetic, environmental, hormonal and inflammatory factors that disrupt glucose homeostasis.12,14,15
1. Initiation: Insulin Resistance:
The early event in T2DM is the development of insulin resistance, a state where peripheral tissues such as skeletal muscle, adipose tissue and the liver become less responsive to insulin. This causes:
● Decreased glucose uptake by muscle cells.
● Reduced inhibition of hepatic glucose production leading to increased glucose release into the bloodstream.
● Elevated levels of free fatty acids (FFAs), further impairing insulin action and contributing to lipotoxicity.
Contributing factors to insulin resistance include:
● Visceral obesity and physical inactivity.
● Accumulation of FFAs and pro-inflammatory cytokines (e.g., TNF-α, IL-6).
● Mitochondrial dysfunction and oxidative stress in adipocytes and muscles.
● Hormonal changes such as those seen during puberty or in stress.
2. Compensation: Hyperinsulinemia:
Initially, in response to insulin resistance pancreatic β-cells increase insulin secretion to maintain normal blood glucose levels (compensatory hyperinsulinemia). However, this overproduction places increasing metabolic stress on the β-cells.
3. β-Cell Dysfunction and Failure:
Over time, pancreatic β-cells begin to fail due to persistent metabolic stress and exposure to glucotoxicity and lipotoxicity. This leads to:
● Impaired insulin synthesis and secretion.
● Increased β-cell apoptosis.
● Oxidative and endoplasmic reticulum (ER) stress.
● Reduction in β-cell mass.
Eventually, insulin levels decline and glucose levels rise persistently, marking the onset of clinical diabetes.
4. Hepatic and Hormonal Dysregulation:
Liver cells continue to produce glucose excessively due to a lack of insulin suppression. Additionally, hormonal dysregulation occurs:
● Glucagon levels are abnormally elevated due to α-cell dysfunction, promoting further hepatic glucose output.
● Incretins (e.g., GLP-1) are deficient or ineffective, leading to impaired postprandial insulin secretion.
● Amylin, co-secreted with insulin, may form toxic aggregates that contribute to β-cell destruction.
5. Mitochondrial and Inflammatory Pathways:
In T2DM, mitochondria fail to effectively metabolize substrates, producing excess reactive oxygen species (ROS), which cause oxidative damage to insulin-responsive tissues and β-cells. Chronic low-grade inflammation also plays a key role:
● Proinflammatory cytokines damage insulin signaling pathways.
● Immune cells infiltrate adipose tissue and the pancreas, further impairing insulin action and secretion.
6. Psychosocial and Epigenetic Factors:
Stress, trauma and adverse childhood experiences increase the risk of T2DM, especially in youth. Stress hormones like cortisol disrupt glucose metabolism and insulin sensitivity. Additionally, epigenetic programming from in utero exposure to maternal diabetes or malnutrition can predispose individuals to β-cell dysfunction and insulin resistance from birth.
7. Accelerated Progression in Youth-Onset T2DM:
In children and adolescents, T2DM progresses 2–3 times faster than in adults. Key features include:
● More severe insulin resistance during puberty.
● Rapid loss of β-cell function despite therapy (as shown in studies like TODAY and RISE).
● Early onset of microvascular (retinopathy, nephropathy) and macrovascular (hypertension, dyslipidemia) complications.
● Higher likelihood of treatment failure with monotherapy, often requiring combination or insulin therapy early.
8.Systemic Effects and Complications
As T2DM progresses, chronic hyperglycemia and associated metabolic disturbances lead to widespread organ damage:
● Cardiovascular system: Accelerated atherosclerosis, hypertension, heart failure.
● Kidneys: Diabetic nephropathy, leading to proteinuria and potential renal failure.
● Eyes: Diabetic retinopathy, macular edema and vision loss.
● Nerves: Peripheral and autonomic neuropathies.
● Liver: Nonalcoholic fatty liver disease (NAFLD).
● Reproductive system: Polycystic ovary syndrome (PCOS) in females; erectile dysfunction in males.
Risk Factors for Diabetes
1. Non-Modifiable Risk Factors
These are risk factors that cannot be changed but play a significant role in susceptibility:
a. Age: Risk increases significantly after age 45 due to declining insulin sensitivity and beta-cell function.
b.Genetics / Family History: Having a first-degree relative (parent or sibling) with diabetes greatly increases the risk.
c.Ethnicity: Higher risk in specific populations:
● African Americans
● Hispanic/Latino Americans
● Native Americans
● Asian Americans
● Pacific Islanders
d. History of Gestational Diabetes: Women with GDM have a 35–60% chance of developing T2DM within 10–20 years post-delivery.
e. Polycystic Ovary Syndrome (PCOS): PCOS is linked to insulin resistance and is an independent risk factor for type 2 diabetes.
2.Modifiable Lifestyle Risk Factors
These are preventable or manageable through behavior and lifestyle changes:
a. Obesity: Especially abdominal obesity; strong association with insulin resistance.
b. Sedentary Lifestyle: Lack of physical activity decreases insulin sensitivity and promotes weight gain.
c. Unhealthy Diet: Diets high in refined carbohydrates, sugars, saturated fats and low in fiber increase diabetes risk.
d. Smoking: Smokers have a 30–40% higher risk of developing type 2 diabetes.
e. Alcohol Consumption: Excessive alcohol disrupts glucose metabolism and contributes to weight gain.
f. Sleep Disturbances: Poor sleep quality, short sleep duration and sleep apnea are associated with glucose intolerance.
g. Chronic Stress: Elevates cortisol and promotes insulin resistance and visceral fat accumulation.
3. Medical Conditions and Medications
Certain conditions or drugs may increase diabetes risk:
a. Cardiovascular Diseases: Conditions like hypertension, atherosclerosis and dyslipidemia often coexist with diabetes.
b. Hormonal Disorders:
● Cushing’s syndrome
● Acromegaly
Both increase blood glucose levels via hormone imbalances.
c. Certain Medications: Long-term use of the following may induce hyperglycemia:
● Corticosteroids
● Atypical antipsychotics
● Some antiretroviral drugs16,17,18,19
Conclusion
Diabetes mellitus is not merely a disorder of blood sugar regulation but a systemic, progressive condition that affects multiple organs and bodily functions. Its onset and progression are influenced by a broad spectrum of interrelated factors including genetic predisposition, lifestyle choices, environmental exposures and underlying medical conditions. As this article has explored, understanding the causes, symptoms, pathophysiological mechanisms, risk factors and complications of diabetes is fundamental in comprehending the full scope of the disease.
Raising awareness about modifiable risk factors such as obesity, sedentary behavior, poor dietary habits and smoking along with early detection through symptom recognition and preventive strategies can dramatically alter the trajectory of the disease. Additionally, a deeper understanding of pathophysiology provides critical insights into the underlying mechanisms that lead to chronic complications offering new avenues for targeted intervention and management. Ultimately, this knowledge forms the cornerstone of effective prevention, personalized care and public health strategies aimed at curbing the global diabetes epidemic. Empowering individuals with this information enables proactive engagement in health maintenance and encourages timely medical consultation.
The next articles in this series will delve into diagnostic approaches and treatment strategies for diabetes mellitus discussing both conventional methods and emerging innovations to support optimized patient care and long-term disease management.
References
- Diabetes. WHO. Published on November 14, 2024. Accessed on May 19, 2025. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes
- Samar A. Antar, Nada A. Ashour, Marwa Sharaky, Muhammad Khattab, Naira A. Ashour, Roaa T. Zaid, Eun Joo Roh, Ahmed Elkamhawy, Ahmed A. Al-Karmalawy. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomedicine & Pharmacotherapy. 2023;168:115734. https://www.sciencedirect.com/science/article/pii/S0753332223015329
- Diabetes. WHO. Accessed on May 19, 2025. Available from: https://www.who.int/health-topics/diabetes#tab=tab_1
- Punthakee, Zubin et al. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Canadian Journal of Diabetes. 2018;42(1):S10 – S15. https://www.canadianjournalofdiabetes.com/article/S1499-2671(17)30813-4/fulltext
- Alam S, Hasan MK, Neaz S, Hussain N, Hossain MF, Rahman T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology. 2021; 2(2):36-50. https://www.mdpi.com/2673-4540/2/2/4
- Diabetes UK. Prediabetes symptoms and risk reduction. Accessed on May 19, 2025. Available from: https://www.diabetes.org.uk/about-diabetes/type-2-diabetes/prediabetes
- Khan RMM, Chua ZJY, Tan JC, Yang Y, Liao Z, Zhao Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina (Kaunas). 2019 Aug 29;55(9):546. https://pmc.ncbi.nlm.nih.gov/articles/PMC6780236/#sec4-medicina-55-00546
- The Lancet Diabetes & Endocrinology. Prediabetes: much more than just a risk factor. The Lancet Diabetes & Endocrinology. 2025;13(3):165. https://www.thelancet.com/journals/landia/article/PIIS2213-8587(25)00034-8/fulltext
- American Diabetes Association Professional Practice Committee; 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 1 January 2024; 47 (Supplement_1): S20–S42. https://diabetesjournals.org/care/article/47/Supplement_1/S20/153954/2-Diagnosis-and-Classification-of-Diabetes
- Ramachandran A. Know the signs and symptoms of diabetes. Indian J Med Res. 2014 Nov;140(5):579-81. https://pmc.ncbi.nlm.nih.gov/articles/PMC4311308/#sec1-2
- American Diabetes Association. Good to Know: Diabetes Symptoms and Tests. Clin Diabetes 1 January 2020; 38 (1): 108. https://diabetesjournals.org/clinical/article/38/1/108/32138/Good-to-Know-Diabetes-Symptoms-and-Tests
- Ozougwu, Jevas & Obimba, K.C. & Belonwu, C.D. & Unakalamba, C.B.. (2013). The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. Academic Journals. 2013;4(4):46-57. https://www.researchgate.net/publication/312716171_The_pathogenesis_and_pathophysiology_of_type_1_and_type_2_diabetes_mellitus
- Federica Del Chierico. et al. Pathophysiology of Type 1 Diabetes and Gut Microbiota Role. J. Mol. Sci. 2022, 23(23), 1465. https://www.mdpi.com/1422-0067/23/23/14650
- Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020 Aug 30;21(17):6275. https://pmc.ncbi.nlm.nih.gov/articles/PMC7503727/#sec3-ijms-21-06275
- Fida Bacha et al. Pathophysiology and Treatment of Prediabetes and Type 2 Diabetes in Youth. Diabetes Care 2024;47(12):2038–2049. https://diabetesjournals.org/care/article/47/12/2038/157188/Pathophysiology-and-Treatment-of-Prediabetes-and
- American Heart Association. Diabetes Risk Factors. Last Reviewed: Apr 3, 2024. Accessed on May 21, 2025. Available from: https://www.heart.org/en/health-topics/diabetes/understand-your-risk-for-diabetes
- Ismail L, Materwala H, Al Kaabi J. Association of risk factors with type 2 diabetes: A systematic review. Comput Struct Biotechnol J. 2021 Mar 10;19:1759-1785. https://pmc.ncbi.nlm.nih.gov/articles/PMC8050730/#s0015
- Arya P. Risk Factors of Diabetes; Review Article. Dia. Med. Care. (2023) 6(3), 61–66. https://www.openaccessjournals.com/articles/risk-factors-of-diabetes.pdf
- Tang SS, Zhao XF, An XD, Sun WJ, Kang XM, Sun YT, Jiang LL, Gao Q, Li ZH, Ji HY, Lian FM. Classification and identification of risk factors for type 2 diabetes. World J Diabetes 2025; 16(2): 100371. https://www.wjgnet.com/1948-9358/full/v16/i2/100371.htm